Meet our Founder @ Rare 2018 in London
Meet our Founder @ Rare 2018 in London

Meet our Founder at Rare 2018 in London!
Hermann is well known in the community, as he found several biomarkers for rare lysosomal diseases e.g. Fabry, Gaucher, NP-C, MLD.
Meet our Founder @ Rare 2018 in London

Meet our Founder at Rare 2018 in London!
Hermann is well known in the community, as he found several biomarkers for rare lysosomal diseases e.g. Fabry, Gaucher, NP-C, MLD.
Meet our new head of Business Development@BIO-EUROPE in Berlin!

Meet our new head of Business Development@BIO-EUROPE in Berlin!
Reinhard received a PhD in Clinical Pharmacology from the University of Vienna (thesis about development of a multi compound HPLC method with semi-automated SPE). He has a long time experience in selling chromatography disposables, instruments and services within Europe.
His goal as the head of business development at pharm-analyt is to work closely with clients for a better understanding for the future needs in the market.

14.00 Derivatisierungsfreier Nachweis von Östrogenen in Plasma im untersten pg/ml Bereich mittels Ionen Mobilität auf einem High-End-QTRAP System, Prof. Hermann Mascher


Bioanalytical Winter Promotion

It has been quite a hot period for us between summer 2015 and summer 2016 here at pharm-analyt. Due to capacity improvements in documentation we have been able to close many projects by fall 2016. The winter season appears to be cooler, for us also in terms of business, and we are anticipating excess capacity between December 2016 and February 2017.
However, we are not even considering hibernating!
Rather, we invite you to join our seasonal promotion: If you order one of the two packages gingerbread or mistletoe between 1 December 2016 and 28 February 2017 you will benefit from our promotional package prices. The GLP assays qualifying for the packages are displayed in a table below:
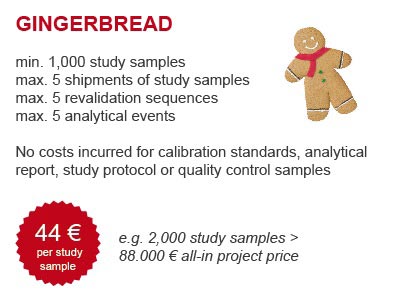

| Allopurinol and Oxipurinol | Human Plasma | 0.1 mL | 2 - 500 ng/mL both |
| Azelaic Acid | Human Plasma | 0.05 mL | 1 - 500 ng/mL |
| Budesonide | Human Plasma | 0.25 mL | 5 - 1000 pg/mL |
| Cortisol | Human Plasma | 0.1 mL | 1.5 - 500 ng/mL |
| Cortisol | Human Serum | 0.1 mL | 1.5 - 500 ng/mL |
| Cortisol | Human Urine | 0.1 mL | 1.5 - 500 ng/mL |
| Cyclosporin A | Whole Blood | 0.05 mL | 0.2 - 100 ng/mL |
| Esmolol | Whole Blood | 0.05 mL | 10 - 5000 ng/mL |
| Fluticasone Propionate | Human Plasma | 1 mL | 0.25 - 50 pg/mL |
| Fluticasone Propionate | Human Serum | 0.25 mL | 3 - 1000 pg/mL |
| Formoterol | Human Plasma | 0.25 mL | 0.4 - 100 pg/mL |
| Landiolol | Whole Blood | 0.05 mL | 10 - 5000 ng/mL |
| Metformin | Human Plasma | 0.05 mL | 10 - 2000 ng/mL |
| Nabilone | Human Plasma | 0.5 mL | 0.05 - 10 ng/mL |
| n-Acetyl-Carnitine | Human Plasma | 0.02 mL | 50 - 10000 ng/mL |
| Nicorandil | Human Plasma | 0.1 mL | 1 - 500 ng/mL |
| Paclitaxel | Human Plasma | 0.05 mL | 8 - 8000 ng/mL |
| Pimelic Acid | Human Plasma | 0.05 mL | 0.5 - 250 ng/mL |
| Raloxifene | Human Plasma | 0.5 mL | 20 - 5000 pg/mL |
| Salmeterol | Human Serum | 0.25 mL | 2 - 600 pg/mL |
| Treprostinil | Human Plasma | 0.25 mL | 4 - 2000 pg/mL |
Note: This is only a small fraction of pharm-analyt's assays
Reflection on Three Decades of Bioanalysis

Reflection on Three Decades of Bioanalysis
As founder and CEO of pharm-analyt I go back a long way.
Back in 1975, when I started in the field of bioanalysis, HPLC was brand new technology and UV detectors with fixed wavelength at 254 nm were just becoming the new standard.
In 1978 I obtained a “SP8000”. Being the “Mercedes” among the HPLC-systems, it featured a ternary gradient(!) and a Schoeffel UV detector with variable wavelength! Coming from GC-technology, it was inconceivable, that most any molecule could be determined – not only evaporable molecules or ones made evaporable through derivatisation. Back then, any new molecule determined in plasma was a little sensation, worth a publication!
In the 1990s HPLC-MS systems with ESI (Electrospray Ionisation) and APCI (Atmospheric Pressure Chemical Ionization) became stable enough to be an option for routine work. This is presuming you had the budget, as the cost of technology was outrageously.
I remember, cleaning and establishing “good vacuum” consumed almost half of our time but at least molecules without chromophores and functional groups for derivatisation (e.g. for fluorescence) now became visible in the low ng/mL range.
In the 1980s, a combination of HPLC with electrochemical detectors and glassy carbon, later with porous graphite, was a brilliant option for molecules that can be oxidised.
Then came mass spec technology. The speed of development of HPLC-MS/MS technology since 1995 is staggering! Analyzing peptides with MS has become routine, not to forget PEGylated drugs, hormones and other formerly “terrible” things!
Of course, the femtogram/mL mark in plasma has been passed a long time ago, and we’re ever continuing to shift the limits!
Being part of this dynamic and innovative world of bioanalysis is such a privilege and we are certainly looking forward to the future!
Join us into a new and exciting decade!
Hermann Mascher
CEO and founder of pharm-analyt
pharm-analyt Labor Gmbh | Ferdinand-Pichler-Gasse 2 | 2500 Baden | AUSTRIA | Copyright © 2026