Investigation
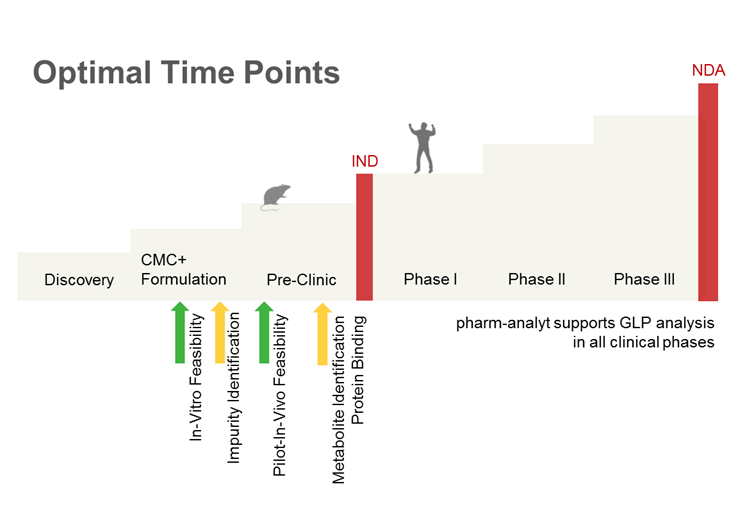
Each project is unique.
Especially when working with a new chemical entity (NCE) we highly recommend to do an early “quick and dirty” feasibility study in “real plasma” to get a grasp of how your compound behaves in plasma (see Feasibilities).
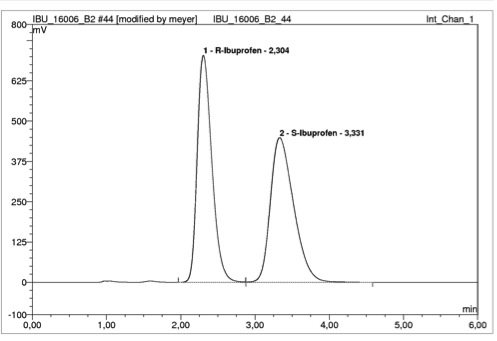
Other questions have to be clarified: Issues with protein binding? Enantio selective specifics?
Tissue analysis is a topic of its own. Done “correctly” it requires a lot of knowledge and experience, especially selection and preparation of tissue samples.